Background: Primary myelofibrosis (PMF) has the worst prognosis of the classical BCR-ABL1 negative myeloproliferative neoplasms, with a median overall survival of six years. Factors affecting survival include age, symptom burden, cytopenias, mutation profile, and development of second malignancies including transformation to acute myeloid leukemia (AML). Ruxolitinib, a selective JAK1 and JAK2 inhibitor, was granted approval by the United States (US) Food and Drug Administration for treatment of intermediate and high-risk PMF in November 2011 based on reduction in spleen volume and demonstration of symptom improvement. The impact of ruxolitinib on PMF survival is unknown. In this study, we aimed to evaluate whether there has been a change in survival and patterns of second primary malignancies (SPMs) including AML transformation among PMF population in US after ruxolitinib approval.
Methods: Using the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER)-18 survival and Multiple Primary Standardized Incidence Ratio (MP-SIR) registries, we conducted a retrospective study with patients diagnosed with PMF between the years of 2007 and 2016. We divided these patients into two five-year cohorts, pre-ruxolitinib approval (2007-2011) and post-ruxolitinib approval (2012-2016), and compared relative survival rates (RSRs) and standardized incidence ratios (SIRs) of SPMs between the cohorts. SIRs were calculated as the ratio of observed to expected malignancy cases over the specified time periods. Median follow-up duration was five years for each cohort. RSRs and SIRs were compared between cohorts using two-proportion Z-tests.
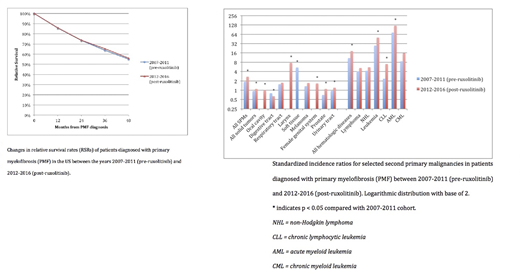
Results: We included 2164 patients diagnosed with PMF between 2007 and 2016 with data available in the SEER-18 survival and MP-SIR registries. Of these, 1051 (49%) patients were included in the pre-ruxolitinib cohort and 1113 (51%) patients were included in the post-ruxolitinib cohort. There was no significant difference in the four-year RSRs between the pre-ruxolitinib and post-ruxolitinib cohorts (55% vs. 56%, p = 0.719). A higher proportion of SPMs occurred in the post-ruxolitinib cohort when compared with the pre-ruxolitinib cohort (60% vs. 40%, p < 0.001). Hematologic malignancies comprised a majority of all SPMs (AML 39% and non-Hodgkin lymphoma 16%). A higher incidence of AML transformation occurred in the post-ruxolitinib cohort when compared with the pre-ruxolitinib cohort (SIR 121.48 vs. 72.22, p = 0.037). Non-hematologic malignancies were also more common in the post-ruxolitinib cohort when compared with the pre-ruxolitinib cohort (SIR 1.09 vs. 0.94, p < 0.001). The most common non-hematologic malignancies were cancers of the respiratory tract, urinary tract, and prostate gland, though their SIRs were not significant in either cohort.
Conclusions: Our study results suggest that despite improvements in prognostication and the approval of ruxolitinib, the prognosis of PMF remains poor in the US. These results may be due to low uptake of ruxolitinib in practice or a lack of benefit from the drug itself. Additionally, for reasons that are unclear, SPM incidence has increased in the five years following the approval of this drug. Further studies should be conducted to determine the cause of these findings.
Shah:Dren Bio: Consultancy. Vachhani:astellas: Speakers Bureau; agios, blueprint medicines, jazz pharmaceuticals, daiichi sankyo: Membership on an entity's Board of Directors or advisory committees; incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.